Abstract
Introduction: Anti-BCMA CAR-T is a highly effective therapy approved for relapsed/refractory multiple myeloma (MM). Its main toxicities include cytokine release syndrome, neurotoxicity, and cytopenias. Infections are common but not well-studied in this population. In this study, we seek to characterize the timing and nature of infections to better inform appropriate prophylactic measures.
Methods: We retrospectively reviewed all MM patients (pts) treated with anti-BCMA CAR-T at Mount Sinai from 2018-2022. Data were collected on baseline characteristics, treatment, disease response, hypogammaglobulinemia (HGG), infections, and prophylactic measures, including intravenous immunoglobulin (IVIg). The study period was from time of CAR-T infusion until progression or death. Chi-squared analysis was used for univariate associations and the Kaplan-Meier method was used for time-to-event calculations. Incidence rates ratios (IRR) were calculated using the exact Poisson method. This study was approved by the Mount Sinai IRB.
Results: 80 pts were treated with anti-BCMA CAR-T; the median age was 61 years (range 35-77) and 40% were female. The median time from diagnosis to treatment was 5.1 years, with a median of 5 prior lines of therapy (range 1-18). 71% had high risk cytogenetics, and pts had a median of 2 infections (range 0-8) in the year prior to CAR-T. IVIg was used prophylactically in 95% of pts, usually for at least the first 6 months, and these pts were on IVIg 49% of the total time on study. All patients received prophylaxis for VZV, PCP (if CD4< 200/mm3), and anti-bacterial plus anti-fungal for ANC <500/mm3.
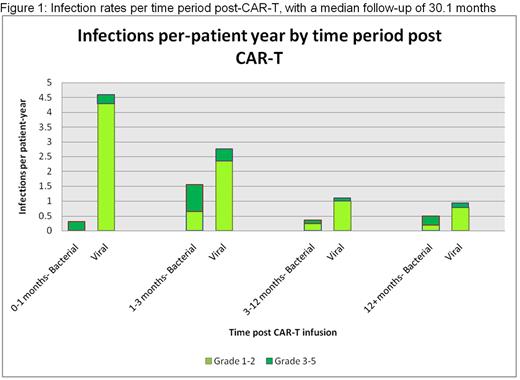
Median follow-up was 30.1 months, with ORR of 94%, median PFS of 18.8 months and estimated OS of 70% at 3 years. CRS occurred in 86% (6% grade 3/4), with tocilizumab used in 73% and steroids in 34%. There were a total of 207 infections during 1336 months of follow-up (1.9 per pt-year), including 57 grade 3-5 infections (0.5 per pt-year) among 25 (31%) pts, and 5 (6%) deaths, with infection rates highest in the first 3 months (Figure 1). Deaths from infection occurred at months 3 (bacterial osteomyelitis), 4.6 (COVID-19), 5.3 (bacterial pneumonia), 24.4 (bacterial skin/soft tissue), and 26.4 (fungemia). Infections (all-grade/ grade 3-5) were mostly in the respiratory tract (63%/43%), followed by GI (11%/32%), skin/soft tissue (5%/11%), and bloodstream (5%/21%), and were mostly viral (68%/38%), followed by bacterial (30%/63%) and fungal (2%/5%). Few opportunistic infections occurred, including grade 3 Cryptosporidiosis, grade 3 Citrobacter sedlakii bacteremia, grade 5 Parabacteroides distasonis abscess and osteomyelitis, and grade 5 Saccharomyces fungemia. Of those with follow-up since 2020, 16 (25%) tested positive for SARS-CoV-2, with 4 severe cases including 1 death at 4.6 months (of these, 2 were vaccinated, including the patient who died). Asymptomatic CMV reactivation occurred in 34% and was only treated in 3 patients.
95% developed HGG, with severity difficult to gauge due to routine IVIg use. Immunoglobulin (Ig) recovery occurred at 14.3 months for one uninvolved Ig and 20 months for two uninvolved Igs. Absolute neutrophil count <500/mm3 and absolute lymphocyte count <500/mm3 were each associated with more severe infections (p<0.001). The 26 pts with 1-3 prior lines of therapy (LoT), compared with those with 4+ LoT, had lower overall infection rate (1.68 per pt-year vs 2.42 per pt-year, IRR 0.70, 95% CI [0.51-0.94], p=0.0.013) and grade 3-5 infection rate (0.29 per pt-year vs 0.62 per pt-year, IRR 0.46, 95% CI [0.21-0.91] p=0.016).
Discussion: Infections among pts treated with anti-BCMA CAR-T were predominantly viral and occurred in the highest rates in the first 3 months. Neutropenia and lymphopenia, common in the first 3 months, were each associated with more severe infections, emphasizing the need for intensive prophylaxis and close outpatient monitoring during this period. Fungal and other opportunistic infections were rare, although one vaccinated patient died of COVID-19, highlighting the need for passive immunity in this population. Ig recovery occurred at 14.3 months, so the optimal duration of IVIg prophylaxis remains an open question. Of note, pts treated in earlier lines of therapy had significantly fewer infections, including grade 3-5 infections.
Disclosures
Lancman:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Cho:Takeda: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding; BMS/Celgene: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding. Richter:Secura Bio: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Takeda: Consultancy; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rodriguez:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rossi:BMS: Consultancy; gsk: Consultancy; sanofi: Consultancy; adaptive: Consultancy; janssen: Consultancy. Jagannath:BMS: Consultancy; Sanofi: Consultancy; Legend Biotech: Consultancy; Janssen Pharmaceuticals: Consultancy; Takeda: Consultancy; Karyopharm: Consultancy. Chari:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Glaxo Smith Klein: Research Funding; Novartis Pharmaceuticals: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Array Biopharma: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncoceutics: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Richard:Karyopharm: Consultancy, Honoraria; C4 Therapeutics: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal